NAFDAC raises alarm over counterfeit Tandak injection sales
By Innocent Raphael
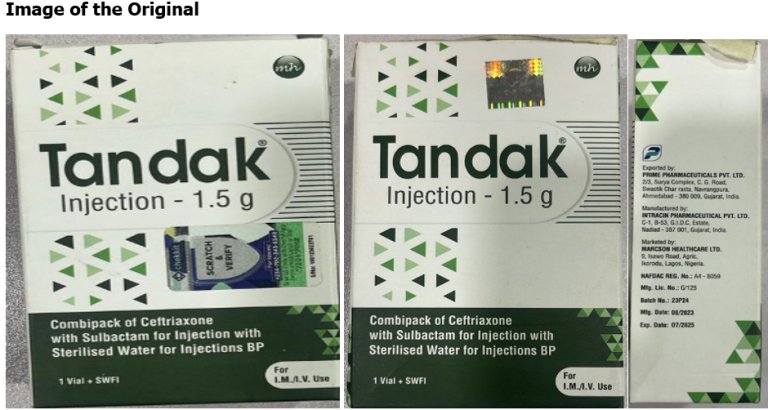
The Nigerian Agency for Food and Drug Administration and Control (NAFDAC) has raised concerns over the sale of counterfeit TANDAK injection 1.5g powder and water for injection in Nigeria.
The agency disclosed this in a statement made public via its X handle on Thursday.
According to NAFDAC, this counterfeit product is manufactured by Intracin Pharmaceuticals PVT. LTD in Gujarat, India, and was discovered in Gombe State and reported by Marcson Healthcare Ltd., the Marketing Authorization Holder (MAH).
Tandak® injection, containing a co-formulation of Ceftriaxone 1000mg and Sulbactam 500mg, is a medication prescribed for treating various bacterial infections. It works by inhibiting the growth and spread of microorganisms.
NAFDAC emphasizes that Ceftriaxone+Sulbactam 1000mg/500mg Injection should only be administered under the supervision of healthcare professionals.
To address the issue, NAFDAC has instructed its zonal directors and state coordinators to conduct surveillance and eliminate the distribution of counterfeit products within their respective zones and states.
NAFDAC also urged healthcare providers and consumers to promptly report any suspicions regarding the sale of substandard or falsified medicines or medical devices.




